When Dmitri Ivanovich Mendeleev formulated his Periodic Law and created his version of the Periodic table, he never imagined that one day the Periodic Table will have more than 100 elements. Mendeleev classified the elements by their increasing Atomic Weights and thus created a table with many gaps of unknown elements. It helped to in the discovery of many unknown elements. Chemistry notebooks are going to be rewritten again.
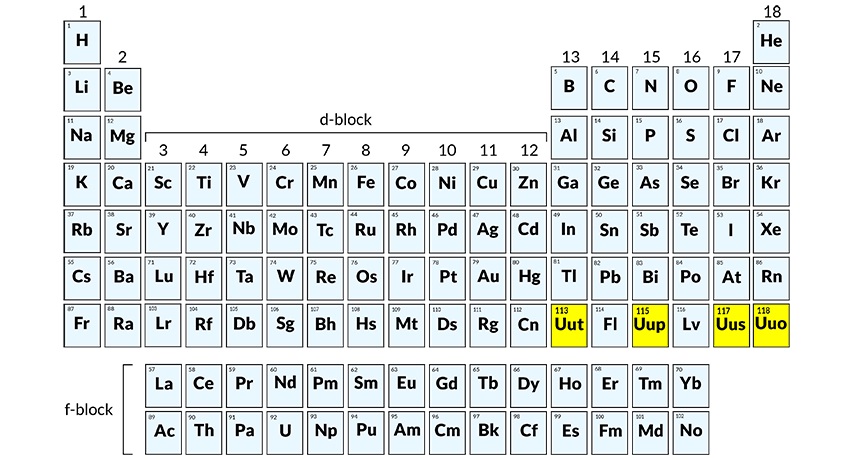
Chemists will have to add four more new elements in their periodic table. As of now they will be known by their working name- ununseptium and ununtrium — two of the four new elements whose discovery has been officially verified. These elements have an atomic number 113, 114, 115, 117, and 118 according to IUPAC and will soon have a new and permanent name soon.
It has been four years since the discovery of elements 114 (flerovium, or Fl) and element 116 (livermorium or Lv) which were then added to the periodic table. The 7th period is complete as per IUPAC.
The elements were discovered in laboratories across Japan, Russia and the United States. The element 113 was discovered by a team at the Riken Institute.
Paul Karol, chair of the IUPAC’s Joint Working Party, announcing the new elements said that it was not an easy task since these new elements decay into completely unknown isotopes.
According to International guidelines for choosing a name, any link to a mythological concept, a mineral, a place or a country, a scientist can be used.
It brings to the question –Why are scientists looking for new elements? The answer is they are hoping to find an element or a series of elements that are stable and useful for people. The quest for new elements also leads to a better understanding of the way atoms are joined and behave in different circumstances.